electron affinity graph|electron affinity of s : Cebu Electron affinity is the energy change that results from adding an electron to a gaseous atom. For example, when a fluorine atom in the gaseous state gains an . All jobs require a clearance, some require a Top Secret. Legal Permanent Residents can get a secret clearance and some 3D AFSC’s only require a secret. I am a recruiter and have enlisted non US citizens into the Air Force with a security clearance. Edit to add: officers must be native born or naturalized to be appointed.
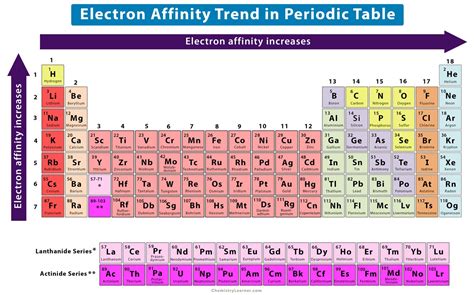
electron affinity graph,Electron affinity is defined as the change in energy (in kJ/mole) of a neutral atom (in the gaseous phase) when an electron is added to the atom to form a negative ion. In other words, the neutral atom's likelihood of gaining an electron.

Learn about dipole moments, a measure of the polarity of a molecule, and how they . Ago 11, 2023
The electron affinity (Eea) of an atom or molecule is defined as the amount of energy released when an electron attaches to a neutral atom or molecule in the gaseous state to form an anion. X(g) + e → X (g) + energyThis differs by sign from the energy change of electron capture ionization. The electron affinity is positive when energy is released on electron capture. Electron affinity is the energy change that results from adding an electron to a gaseous atom. For example, when a fluorine atom in the gaseous state gains an .
Electron affinity can be defined in two equivalent ways. First, as the energy .For example, the first electron affinity of oxygen is −141 kJ/mol, but the second electron affinity is +744 kJ/mol: \[O_{(g)} + e^- \rightarrow O^-_{(g)} \;\;\; EA_1=-141 \;kJ/mol .
What is Electron Affinity. The electron affinity is defined as the amount of energy released per mole when an electron is added to a neutral atom. It is the opposite of ionization energy [1-4]. How to Find Electron .
Electron Affinities. Electron affinity, often abbreviated as EA, is the energy released when an electron is added to a valence shell of the atom. F(g) + e - -> F-(g) EA . Electron affinity is a measure of how readily a neutral atom gains an electron. Electron affinity ( Eea) is the energy change when an electron is added to a neutral atom in the gas phase. In simple .Learn what electron affinity is, how it differs from electronegativity and ionization energy, and how it varies across the periodic table. See a graph of electron affinity values and factors that influence them.Chemists define electron affinity as the change in energy, measured in units of kJ/mole, experienced when an electron is added to a gaseous atom. This process creates a negative ion. This process differs from .
This page deals with the electron affinity as a property of isolated atoms or molecules (i.e. in the gas phase). Solid state electron affinities are not listed here. Elements Electron affinity can be defined in two equivalent ways. First, as the energy that is released by adding an electron to an isolated gaseous atom. The second (reverse) definition is that .electron affinity graph electron affinity of sThe electron affinity is the potential energy change of the atom when an electron is added to a neutral gaseous atom to form a negative ion. So the more negative the electron affinity the more favourable the . Chart of Periodic Table Trends. Use this chart to see at a glance the periodic table trends of electronegativity , ionization energy , atomic radius , metallic character, and electron affinity. Elements are grouped according to similar electronic structure, which makes these recurring element properties readily apparent in the . The opposite of IE is described by electron affinity (EA), which is the energy change when a gas-phase atom accepts an electron: \[A(g)+e^{-}\rightarrow A^{-}(g)\; \; \; \; \; \Delta H\equiv EA \nonumber \]. EA is also usually expressed in kJ/mol. EA also demonstrates some periodic trends, although they are less obvious than the other .The electron affinity of an element is the energy given off when a neutral atom in the gas phase gains an extra electron to form a negatively charged ion. A fluorine atom in the gas phase, for example, gives off energy when it gains an electron to form a fluoride ion. F ( g) + e - F - ( g) Ho = -328.0 kJ/mol. The electron affinity ( EA) is the energy released to add an electron to an elements in the gaseous state. In general successive electron affinity increase in magnitude EA1 < EA2 < EA3 and so on. First electron affinity increases across the period. First electron affinity decreases down the group. The electron affinity of an element is defined as the energy released when an electron is added to an isolated gaseous atom to form a gaseous anion, or negative ion. Usually, only one electron is added, forming a uninegative ion. Since energy is evolved, these terms have a negative sign.
Electron Affinity. In most cases, the formation of an anion by the addition of an electron to a neutral atom releases energy. This can be shown for the chloride ion formation below: Cl + e- → Cl - + energy. The energy change that occurs when a neutral atom gains an electron is called its electron affinity. When energy is released in a .

Figure 4.4.1 graphs the relationship between the first ionization energy and the atomic number of several elements. . Electron affinity (the energy associated with forming an anion) is more favorable (exothermic) when electrons are placed into lower energy orbitals, closer to the nucleus. Therefore, electron affinity becomes increasingly .The electron affinity [EA] is the energy change for the process of adding an electron to a gaseous atom to form an anion (negative ion). X(g) +e− X−(g) EA1 (3.4.1) (3.4.1) X ( g) + e − X − ( g) EA 1. This process can be either endothermic or exothermic, depending on the element. The EA of some of the elements is given in Figure 3.4.6 3.4.
Electron affinity can be defined as the energy required when an electron is removed from a gaseous anion. The reaction as shown in equation 2.3.2.1 2.3.2.1 is endothermic (positive ΔU Δ U) for elements except noble gases and alkaline earth metals. Under this definition, the more positive the EA value, the higher an atom's affinity for electrons.electron affinity of sElectron affinity can be defined in two equivalent ways. First, as the energy that is released by adding an electron to an isolated gaseous atom. The second (reverse) definition is that electron affinity is the energy required to remove an electron from a singly charged gaseous negative ion. The latter can be regarded as the ionization energy .The electron affinity ( EA E A) of an element E E is defined as the energy change that occurs when an electron is added to a gaseous atom or ion: E(g) +e− → E−(g) energy change=EA (7.5.1) (7.5.1) E ( g) + e − → E ( g) − energy change= E A. Unlike ionization energies, which are always positive for a neutral atom because energy is .
Figure \(\PageIndex{3}\): Graph showing the Ionization Energy of the Elements from Hydrogen to Argon. . Electron affinity generally decreases down a group of elements because each atom is larger than the atom above it (this is the atomic radius trend, discussed below). This means that an added electron is further away from the .Electron affinities down a group. Electron affinities generally decrease down a group; As the atoms become larger the attraction for an additional electron is less, since the effective nuclear charge is reduced due to increased shielding; Electron affinity become less exothermic going down the group; An exception to this is fluorine whose electron .
However, when we graph the first ionisation energies for the first 20 elements: There is a general trend upwards across a period, but there are two points of inflexion (changes of direction) in both period 2 and 3. . The electron affinity is defined as the energy change when 1 mole of gaseous negative ions is formed from 1 mole of gaseous .
THe electron affinity is the nergy required to detach an electron from the singly charged negative ion (energy for the process X -> X + e). The equivalent more common definition is the energy released (E initial + E final) when an additional electron is attached to a neutral atom or molecule.[IUPAC Compendium of Chemical Terminology (Gold Book), 2nd .
electron affinity graph|electron affinity of s
PH0 · trend for electron affinity
PH1 · how to tell electron affinity
PH2 · electron affinity vs electronegativity
PH3 · electron affinity trend periodic table
PH4 · electron affinity trend and exceptions
PH5 · electron affinity of s
PH6 · electron affinity of elements
PH7 · electron affinity of cl
PH8 · Iba pa